Abstract
Introduction
Pediatric hospital-acquired venous thromboembolism (HA-VTE) rates are increasing along with associated mortality, short- and long-term morbidity, and healthcare costs. National process improvement efforts are underway to decrease HA-VTE in children, but risk-stratified prevention strategies will be safest and most effective if informed by robust risk factor data. The objective of this work was to identify risk factors for HA-VTE in children via a multicenter study
Methods
The Children's Hospital-Acquired Thrombosis (CHAT) Registry is a multi-institutional pediatric HA-VTE registry that we created to generate a risk-prediction model for future use in clinical trials of thromboprophylaxis. Designed as a retrospective case-control study, CHAT Registry data has been entered by 7 U.S. pediatric institutions for subjects admitted between January 1, 2012 and December 31, 2016. Cases (aged 0-21 years) that developed HA-VTE during the admission were matched by institution and admission year with 1:1 frequency to randomly sampled non-HA-VTE controls.
Univariate analyses tested associations between risk factors and the development of HA-VTE. Analyses utilized weighted logistic regression, in which controls were weighted by the inverse sampling probability in order to recreate the population from which the controls were sampled. Year of admission and hospital sites were included in the assessment of each predictor to control for effects of time and treating hospital.
Results
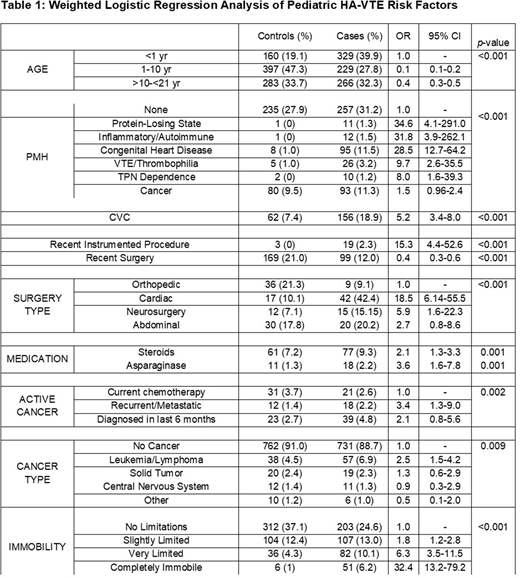
825 HA-VTE cases and 841 controls from participating hospitals comprise the analytical cohort (total 1,666 hospital admissions). HA-VTE cases showed a male predominance (58%). The majority, 73%, were related to central venous catheters (CVCs). HA-VTE most commonly occurred in the lower extremity (32%) followed by upper extremity (16%), thorax/neck (16%), cerebral sinovenous (5%), abdomen (5%), lung (3%), cardiac (2%), and other (22%). VTE were asymptomatic or identified incidentally in 21% of cases. Subjects were most commonly located in an intensive care unit (ICU) (58%) when diagnosed with a VTE, followed by general medical ward (23%), hematology/oncology unit (13%), surgical unit (3%), emergency department (1%), and other (1%).
Significant association with HA-VTE incidence (Table 1) was found with infancy compared to other age groups, prior hospitalization within 1 month (Odds Ratio [OR] 3.6, 95% Confidence Interval [CI] 2.7-4.8) compared to no recent prior admission, admission to ICU (cardiac/pediatric ICU OR 10.1, 95% CI 6.0-17.1 and NICU OR 9.0, 95% CI 5.2-15.7) or hematology/oncology units (OR 2.7, 95% CI 1.6-6.0) compared to admission to general pediatric ward, complete immobility acutely (OR 32.3, 95% CI 13.2-79.2 ) compared to no loss of mobility from baseline, chronic bed-bound/wheelchair-bound immobility (OR 2.2, 95% CI 1.4-3.6), and a past medical history of protein-losing state (OR 34.6, 95% CI 4.1-290.9), inflammatory/autoimmune disease (OR 31.8, 95% CI 3.9-262.1), congenital heart disease (OR 28.5, 95% CI 12.7-64.2), central venous catheter placed on the day of admission (OR 22.0, 95% CI 14.2-34.1) or prior to admission (OR 5.2, 95% CI 3.4-7.9) thrombophilia/VTE (OR 9.7, 95% CI 2.6-35.5), TPN dependence (OR 8.0, 95% CI 1.6-39.3), or history of cancer (OR 1.5, 95% CI 1.0-2.4) compared to the absence of past medical history. Additionally, active cancer conferred an overall odds ratio of 2.0, with the recurrent/metastatic subtype conferring an OR of 3.4 compared to other active cancer subtypes.
Conclusions
The multi-institution CHAT Registry contains a larger dataset of pediatric HA-VTE cases and controls than any similar collection published to date. Key demographic and clinical factors associated with HA-VTE include infant age, male sex, ICU admission, central venous catheter, decreased mobility, cancer, use of certain medications, or certain past medical history (protein-losing state, congenital heart disease, inflammatory/autoimmune condition, thrombophilia or history of VTE, and TPN dependence).
Further analysis is being performed on these and other risk factors to develop a pediatric-specific HA-VTE risk-prediction model for future validation in an even larger multi-institutional cohort. This validated risk model will then be used to identify children at high risk of HA-VTE to guide preventative strategies to decrease the incidence in the pediatric population.
Jaffray:CSL Behring: Consultancy, Research Funding; Octapharma: Consultancy; Bayer: Consultancy. Croteau:Tremeau Pharmaceuticals: Consultancy; Baxalta/Shire: Consultancy, Research Funding; Biomarin: Consultancy; Bioveritiv: Consultancy; Catalyst Biosciences: Consultancy; CSL-Behring: Consultancy; Genetech: Consultancy, Research Funding; Novo Nordisk: Consultancy; Octapharma: Consultancy, Honoraria, Research Funding; Pfizer: Research Funding; Spark Therapeutics: Research Funding; Bayer: Consultancy. Silvey:Bayer: Honoraria; Pfizer: Honoraria; CSL Behring: Honoraria; Octapharma: Honoraria. Young:Bioverativ: Consultancy, Honoraria; Bayer: Consultancy; CSL Behring: Consultancy, Honoraria; Genentech/Roche: Consultancy, Honoraria; Kedrion: Consultancy; Novo Nordisk: Consultancy, Honoraria; Shire: Consultancy, Honoraria. Mahajerin:Genentech Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal